History:
The liposomes were discovered in the 1960s by British biophysicist Alec Bungham.
They were first used as artificial models of cell membranes.
They were used to simulate simple cell systems to perform different types of research.
How liposomal technology is used in supplements?
Liposomal technology is used to facilitate the liposome as a carrier of active ingredients in drugs or supplements, particularly for active ingredients with low absorption in the digestive system.
This unique patented technology makes sure the liposome is naturally diffused in the cell membrane and enhances the active ingredients absorption rate significantly, in any form, whether in a liquid product, in capsules or tablets.
The significant advantage of liposomal products is the natural lipids coating around the ingredients which increases the stability of the supplement, and prevents its breakdown in the acidic environment of the stomach, thus carrying it in a protected manner throughout the entire digestive system until it is properly absorbed in its destination.
In addition, because of the natural coating, high doses of the supplement (such as vitamin C when catching a cold) can be used without causing gastrointestinal irritation and other inconveniences.
Liposomes composition and mechanism formation:
Liposomes are made up of phospholipids – natural molecules with a unique structure that allows them to be both hydrophilic and lipophilic at the same time.
When phospholipids are injected into an aqueous environment, due to the unique combination of oil and water-soluble components, they instantly organize themselves into globular structures (fatty bubbles).
As mentioned before, the various potential use of liposomes is derived from the unique properties of the phospholipids which are molecules with hydrophilic (water-binding) and hydrophobic (water repellent).
When in contact with water, the phospholipids immediately crystallize into a globular structure called colloid.
The hydrophilic phospholipid head binds with the water molecules, while the hydrophobic phospholipid tail moves away from the liquid and to the bubble core’s center, thus creating the characteristic liposomal structure.
This unique structure of the liposomes allows capturing water-soluble components (such as vitamin C and vitamin B12) in their inner core, while the two-layer hydrophobic layer traps oil-soluble components (such as curcumin and coenzyme Q10).
Thanks to this exceptional ability, the liposomes have become a popular carrier in the pharmaceutical and dietary supplements industry.
The absorption of nutrients in the small intestine is occurring in cells named enterocytes, which are covered with microvilli absorption structures.
Liposomal Bioavailability:
The substantially increased amount of the active ingredients of liposomal drugs or dietary supplements in the blood-stream is the evidence for absorption improvement increased bioavailability of high-quality liposomal products.
For this reason, we conducted pharmacokinetic studies, which indicate the level of absorption and biological availability of different products:
From a scientific standpoint, the absorption rate of certain components consumed in swallowing decreases as the supplementation dose is higher. For instance, the common side effect of high vitamin C doses is osmotic diarrhea, caused by the accumulation of excessive fluids in the digestive system.
Therefore, the highest tolerable dose of ingestion of vitamin C is 3-4 grams. Surprisingly, studies in pharmacokinetics (a field that examines the movement and metabolism of a drug or supplement in the body after ingestion) show that supplementing vitamin C in dosages less than 100 mg per day sharply increases its blood concentration.
The range of 100 mg – 400 mg/day, gradually decreases the concentration of vitamin C in the blood-stream until the final measurement of a low concentration measuring 70-80 pmol / L, doses greater than 400 mg per day that result in only a slight increase of vitamin C concentrations in the blood-stream.
Using vitamins produced by liposomal technology enables the administration of high doses while improving vitamin absorption and increase the vitamin concentration in the blood-stream.
For instance, in two patients who took 36 mg of liposomal vitamin C, the concentration in the blood of the vitamin increased to more than 500 pmol / L – a level that can usually be achieved only by intravenous administration.
What are the indicators of a high-quality liposomal product?
- A stable liposomal composition – a combination of a natural molecule of phosphatidylcholine that ensures stable liposome production, and the use of artificial components such as PEG.
- Uniformly minimized particle – the size of the liposome is a critical factor in determining its half-life cycle (duration of survival).
- Liposomes smaller than 100 nm remain active for a longer time, so their potential as absorption enhancers is higher.
- Also, the immune system has increased difficulties in identifying and dissolving (in a process called opsonization) liposomes smaller than 100 nanometers, another fact that helps prolong their activity.
- NO genetic engineering– it is necessary to use high-quality phospholipids and phosphatidyl-choline from non-genetically engineered sources.
- Use of high-quality active ingredients ensures high-quality products hence most effective results.
- Products supported by stability studies and bioavailability studies.
Pharmacokinetic study results: Liposomal vitamin C
Results of the study graphically:
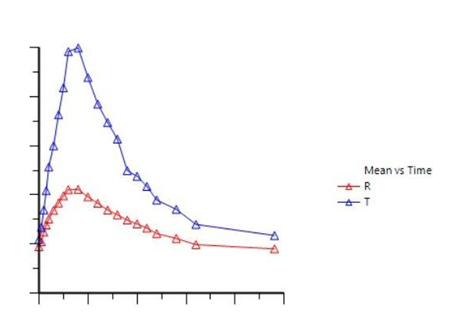
The blue curve describes the liposomal vitamin C level measured in the blood, while the red curve describes the level of non-liposomal vitamin C in the blood.
The study results indicate that the potential for liposomal vitamin C oral administration is an appropriate alternative to intravenous vitamin C treatment.
Liposomal Products Analysis
providing conclusive evidence for the product’s stability, EcoSupp conducted analyses on four products – two of which contain active water-soluble materials, and two other product containing active oil-soluble materials.
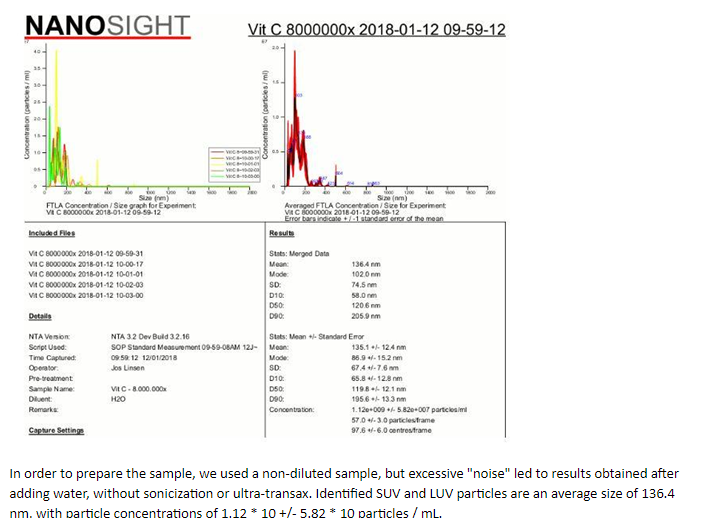
The analysis was carried out in an external laboratory using nanoscale laser scanning instruments: NanoSight LM20, which identifies the particles and follows their Brownian motion to determine their size.
test results: